
Molecular and Clinical Characterization of a Founder Mutation Causing G6PC3 Deficiency
Xin Zhen, Michael J. Betti, Meltem Ece Kars, Andrew R. Patterson, Edgar Alejandro Medina-Torres, Selma Cecilia Scheffler Mendoza, Diana Andrea Herrera Sánchez, Gabriela Lopez-Herrera, Yevgeniya Svyryd, Osvaldo M. Mutchinick, Eric R. Gamazon, Jeffrey C. Rathmell, Yuval Itan, Janet Markle, Patricia O’Farrill Romanillos, Saul Oswaldo Lugo-Reyes and Ruben Martinez-Barricarte
Journal of Clinical Immunology, 2024; 45 (1): 53.
G6PC3 deficiency is a monogenic immunometabolic disorder that causes severe congenital neutropenia type 4. Patients display heterogeneous extra-hematological manifestations, contributing to delayed diagnosis. Here, we investigated the origin and functional consequence of the G6PC3 c.210delC variant found in patients of Mexican descent. Based on the shared haplotypes amongst mutation carriers, we estimated that this variant originated from a founder effect in a common ancestor. Furthermore, by ancestry analysis, we concluded that it appeared in the indigenous Mexican population. At the protein level, we showed that this frameshift mutation leads to an aberrant protein expression in overexpression and patient-derived Epstein-Barr Virus-immortalized B (EBV-B) cells. The neutropenia observed in G6PC3-deficient patients is driven by the intracellular accumulation of the metabolite 1,5-anhydroglucitol-6-phosphate (1,5-AG6P) that inhibits glycolysis. We characterized how the c.210delC variant impacts glycolysis by performing extracellular flux assays on patient-derived EBV-B cells. When treated with 1,5-anhydroglucitol (1,5-AG), the precursor to 1,5-AG6P, patient cells exhibited markedly reduced engagement of glycolysis. Finally, we compared the clinical presentation of patients with the mutation c.210delC and all other G6PC3-deficient patients reported in the literature, and we found that the c.210delC carriers display all prominent clinical features observed in prior patients. In conclusion, G6PC3 c.210delC is a loss-of-function mutation that arose from a founder effect in the indigenous Mexican population. These findings may facilitate the diagnosis of additional patients in this geographical area. Moreover, the in vitro 1,5-AG-dependent functional assay used in our study could be employed to assess the pathogenicity of additional G6PC3 variants.
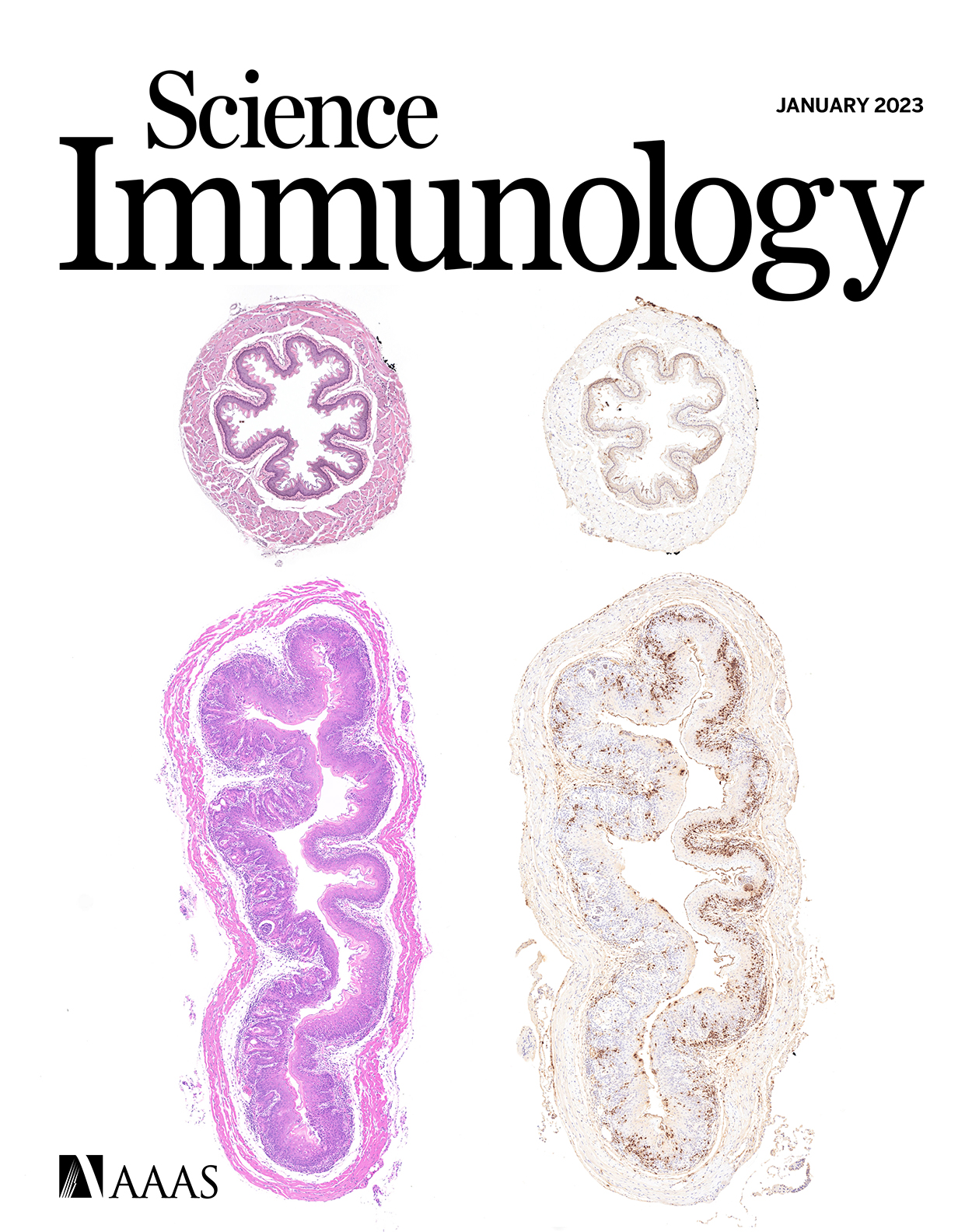
A multimorphic mutation in IRF4 causes human autosomal dominant combined immunodeficiency
IRF4 International Consortium
Science Immunology, 2023; 8 (79): eade7953.
Interferon regulatory factor 4 (IRF4) is a transcription factor (TF) and key regulator of immune cell development and function. We report a recurrent heterozygous mutation in IRF4, p.T95R, causing an autosomal dominant combined immunodeficiency (CID) in seven patients from six unrelated families. The patients exhibited profound susceptibility to opportunistic infections, notably Pneumocystis jirovecii, and presented with agammaglobulinemia. Patients' B cells showed impaired maturation, decreased immunoglobulin isotype switching, and defective plasma cell differentiation, whereas their T cells contained reduced TH17 and TFH populations and exhibited decreased cytokine production. A knock-in mouse model of heterozygous T95R showed a severe defect in antibody production both at the steady state and after immunization with different types of antigens, consistent with the CID observed in these patients. The IRF4T95R variant maps to the TF's DNA binding domain, alters its canonical DNA binding specificities, and results in a simultaneous multimorphic combination of loss, gain, and new functions for IRF4. IRF4T95R behaved as a gain-of-function hypermorph by binding to DNA with higher affinity than IRF4WT. Despite this increased affinity for DNA, the transcriptional activity on IRF4 canonical genes was reduced, showcasing a hypomorphic activity of IRF4T95R. Simultaneously, IRF4T95R functions as a neomorph by binding to noncanonical DNA sites to alter the gene expression profile, including the transcription of genes exclusively induced by IRF44T95R but not by IRF4WT. This previously undescribed multimorphic IRF4 pathophysiology disrupts normal lymphocyte biology, causing human disease.

Clinical and Immunological Features of Human BCL10 Deficiency
Garcia Solis B#, Van Den Rym A#, Pérez-Caraballo JJ#, Al –Ayoubi A, Alazami AM, Lorenzo L, Cubillos-Zapata C, López-Collazo E, Perez-Martinez A, Allende LM, Markle J, Fernandez-Arquero M, Sánchez-Ramón S, Recio MJ, Casanova JL, Mohammed R, Martinez-Barricarte R*, Pérez de Diego R*
Frontiers in Immunology, 2021
The CARD-BCL10-MALT1 (CBM) complex is critical for the proper assembly of human immune responses. The clinical and immunological consequences of deficiencies in some of its components such as CARD9, CARD11, and MALT1 have been elucidated in detail. However, the scarcity of BCL10 deficient patients has prevented gaining detailed knowledge about this genetic disease. Only two patients with BCL10 deficiency have been reported to date. Here we provide an in-depth description of an additional patient with autosomal recessive complete BCL10 deficiency caused by a nonsense mutation that leads to a loss of expression (K63X). Using mass cytometry coupled with unsupervised clustering and machine learning computational methods, we obtained a thorough characterization of the consequences of BCL10 deficiency in different populations of leukocytes. We showed that in addition to the near absence of memory B and T cells previously reported, this patient displays a reduction in NK, γδT, Tregs, and TFH cells. The patient had recurrent respiratory infections since early childhood, and showed a family history of lethal severe infectious diseases. Fortunately, hematopoietic stem-cell transplantation (HSCT) cured her. Overall, this report highlights the importance of early genetic diagnosis for the management of BCL10 deficient patients and HSCT as the recommended treatment to cure this disease.

Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency?
Martínez-Barricarte R*
Frontiers in Immunology, 2020
Nocardiosis is an infectious disease caused by the gram-positive bacterium Nocardia spp. Although it is commonly accepted that exposure to Nocardia is almost universal, only a small fraction of exposed individuals develop the disease, while the vast majority remain healthy. Nocardiosis has been described as an “opportunistic” disease of immunocompromised patients, suggesting that exposure to the pathogen is necessary, but a host predisposition is also required. Interestingly, increasing numbers of nocardiosis cases in individuals without any detected risk factors, i.e., without overt immunodeficiency, are being reported. Furthermore, a growing body of evidence have shown that selective susceptibility to a specific pathogen can be caused by a primary immunodeficiency (PID). This raises the question of whether an undiagnosed PID may cause nocardiosis affecting otherwise healthy individuals. This review summarizes the specific clinical and microbiological characteristics of patients with isolated nocardiosis published during the past 30 years. Furthermore, it gives an overview of the known human immune mechanisms to fend off Nocardia spp. obtained from the study of PIDs and patients under immunomodulatory therapies.
Para ver la lista completa de publicaciones haga click aquí
Martínez-Barricarte R, Kong XF, Casanova JL. (2019). “Measurement of CD74 N-terminal fragment accumulation in cells treated with SPPL2a inhibitor”. Bio-protocols. 9(11):e3254. Protocolo completo
Martínez-Barricarte R, de Jong SJ, Markle J, de Paus R, Boissin-Dupuis S, Bustamante J, van de Vosse E, Fleckenstein B, Casanova JL. (2016). “Transduction of herpesvirus saimiri-transformed T cells with exogenous genes of interest”. Current Protocols in Immunology. 115:7.21C.1-7.21C.12. Protocolo completo
Alsina L, Rodriguez-Gallego C, Esteve-Solé A, Vlagea A, Pérez de Diego R, Martínez-Barricarte R and Deyà-Martínez A. Defects in Intrinsic and Innate Immunity. (2021). Cellular Primary Immunodeficiencies. Springer. 177-212. Libro completo.